Abstract
On Behalf of the European Mantle Cell Lymphoma Network
Background: Mantle cell lymphoma (MCL) was usually characterized by a poor long term outcome. Starting in 2004, the European MCL Network has performed the randomized phase 3 MCL Younger trial for first-line treatment of patients with MCL in Ann-Arbor stage II-IV, aged < 66 years and suitable for autologous stem cell transplantation (Hermine et al., Lancet 2016). In this protocol, efficacy and safety of an alternating R-CHOP/R-DHAP induction followed by an ARA-C containing high dose consolidation with autologous blood stem cell transplantation (ASCT) was compared to R-CHOP followed by myeloablative radio-chemotherapy and ASCT. In the initial analysis, the primary outcome time to treatment failure (TTF) was significantly prolonged in the ARA-C arm (5 years rate 65% vs 40%; p=0.038). Now we report long term outcome of patients after a median follow up of 11 years with a focus on overall survival (OS) differences.
Methods: Primary evaluation of TTF was performed according to a pre-planned modified intention to treat (ITT, mITT) strategy with correction for interim analyses (overrunning analysis); all other efficacy analyses are according to strict ITT. TTF and OS were described by Kaplan-Meier estimates and compared by two-sided log-rank tests. The trial was not powered to detect unadjusted OS differences; accordingly, in the present evaluation, the number of deaths yields a statistical power of 80% and 90% for overoptimistic OS hazard ratios of 0.67 and 0.63. Hazard ratios of R-DHAP vs. R-CHOP with two-sided 95% CI and the corresponding p values were calculated from a univariate Cox proportional hazards model and two multivariate Cox proportional hazards models, one adjusting for MIPI score, and the other adjusting for MIPI score and Ki-67 index, the two components of MIPI-c. We additionally evaluated cumulative incidence of secondary hematological malignancies, treating death without secondary hematological malignancy as competing event.
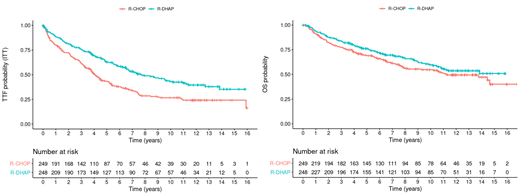
Results: Of 497 patients randomized and evaluable according to ITT, 466 were included in primary evaluation. Median patient age was 55 years (range, 30-67), with MIPI and Ki-67 similar in two arms (MIPI Low 65%/60%, Intermediate 22%/26%, High 13%/14%; Ki-67≥30%: 28%/27%). In primary mITT analysis, TTF was still significant (p=0.038, HR: 0.59, both corrected for interim analyses). Differences in TTF were also confirmed in strict ITT analyses (Figure left; HRs unadjusted/adjusted for MIPI score/adjusted for MIPI-score and Ki-67: 0.60 (95% CI, 0.47-0.76)/0.56 (0.44-0.71)/0.52 (0.38-0.70), all p<0.0001). Median OS was not reached in the R-DHAP arm vs 11.3 years in the R-CHOP arm (Figure right, p=0.12), with 5/10-year OS probabilities of 76%/60% (R-DHAP) and 69%/55% (R-CHOP), respectively, and an unadjusted hazard ratio of 0.80 (95% CI 0.61-1.06, p=0.12). When adjusted for MIPI score without and with Ki-67, OS was significantly superior in the R-DHAP arm (HR 0.74, 95% CI 0.56-0.98, p=0.038 and 0.60, 0.41-0.87, p=0.0066). Although not statistically significant, the cumulative incidence of secondary hematological malignancies was higher in the R-DHAP arm (9 vs. 4 events; at 10 years 4.5% vs 1.4%, p=0.14).
Conclusions: With additional 5 years of median follow-up, our results on first-line treatment of MCL patients younger than 66 years confirm the previously observed substantially prolonged TTF by the addition of high-dose ARA-C. When adjusting for MIPI without and with Ki-67 (conditional treatment effect), OS was significantly prolonged. In the future, avoidance of TBI and ASCT, as investigated in the TRIANGLE protocol, may reduce secondary malignancies after R-CHOP/R-DHAP. These data suggest that some patients may be functionally cured by optimal first line treatment and may challenge future chemotherapy-free strategies in MCL.
Walewski: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Thieblemont: Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Salles: Epizyme: Consultancy, Honoraria; Velosbio: Consultancy; Loxo: Consultancy; Genmab: Consultancy; Incyte: Consultancy; Ipsen: Consultancy; Kite/Gilead: Consultancy; Janssen: Consultancy; Genentech/Roche: Consultancy; Miltneiy: Consultancy; Morphosys: Consultancy, Honoraria; Rapt: Consultancy; Novartis: Consultancy; Allogene: Consultancy; Debiopharm: Consultancy; Takeda: Consultancy; Regeneron: Consultancy, Honoraria; BMS/Celgene: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Honoraria; Bayer: Honoraria. Feugier: ROCHE: Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding. Hübel: Celgene: Consultancy; Gilead: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; EUSA: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau. Klapper: Takeda: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; Amgen: Research Funding; Roche: Consultancy, Research Funding. Unterhalt: Roche: Research Funding. Dreyling: Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Gilead Kite: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Astra Zeneca: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; AbbVie: Research Funding; BeiGene: Consultancy, Speakers Bureau; Genmab: Consultancy; MorphoSys: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal